What Carbonyl Compound Would You Reduce to Produce the Following Alcohol?
17.4 Alcohols from Carbonyl Compounds: Reduction
- Page ID
- 90941
Objectives
After completing this section, y'all should be able to
- decide whether a given reaction should be classified as an oxidation or a reduction.
- write an equation to represent the reduction of an aldehyde or ketone using sodium borohydride or lithium aluminum hydride.
- hash out the relative advantages and disadvantages of using sodium borohydride or lithium aluminum hydride to reduce aldehydes or ketones to alcohols.
- identify the production formed from the reduction of a given aldehyde or ketone.
- identify the aldehyde or ketone that should be used to produce a given alcohol in a reduction reaction.
- identify the best reagent to conduct out the reduction of a given aldehyde or ketone.
- write an equation to represent the reduction of an ester or a carboxylic acid to an alcohol.
- place the product formed from the reduction of a given ester or carboxylic acid.
- place the esters or carboxylic acids that could be reduced to form a given booze.
Primal Terms
Make sure that you can define, and use in context, the key terms below.
- (organic) oxidation
- (organic) reduction
Report Notes
In your class in first‑year general chemistry, yous probably discussed oxidation‑reduction reactions in terms of the transfer of electrons and changes in oxidation numbers (oxidation states). In organic chemistry, it is often more user-friendly to regard reduction as the gain of hydrogen or loss of oxygen, and oxidation as the proceeds of oxygen or the loss of hydrogen. There is no contradiction in using these various definitions. For example, when hydrogen is added across the double bond of ethene to reduce it to ethane, the oxidation number of the doubly bonded carbon atoms decreases from −2 to −III. Similarly, when ii‑propanol
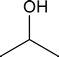
is oxidized to acetone
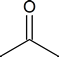
hydrogen is removed from the compound and the oxidation number of the central carbon atom increases from 0 to +II. If necessary, review the concept of oxidation number.
Reduction of Aldehydes and Ketones
The most common sources of the hydride Nucleophile are lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4). Note! The hydride anion is not nowadays during this reaction; rather, these reagents serve as a source of hydride due to the presence of a polar metallic-hydrogen bond. Because aluminum is less electronegative than boron, the Al-H bond in LiAlHfour is more polar, thereby, making LiAlH4 a stronger reducing agent.
Add-on of a hydride anion (H:-) to an aldehyde or ketone gives an alkoxide anion, which on protonation yields the respective booze. Aldehydes produce 1º-alcohols and ketones produce 2º-alcohols.
In metal hydrides reductions the resulting alkoxide salts are insoluble and need to be hydrolyzed (with care) before the alcohol production can be isolated. In the sodium borohydride reduction the methanol solvent arrangement achieves this hydrolysis automatically. In the lithium aluminum hydride reduction water is usually added in a 2nd step. The lithium, sodium, boron and aluminum end upwardly as soluble inorganic salts at the terminate of either reaction. Note! LiAlH4 and NaBH4 are both capable of reducing aldehydes and ketones to the corresponding alcohol.
Example 17.4.1
Mechanism
This mechanism is for a LiAlH4 reduction. The mechanism for a NaBHiv reduction is the same except methanol is the proton source used in the second step.
1) Nucleopilic attack by the hydride anion
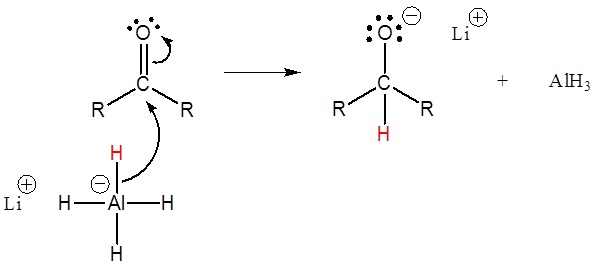
ii) The alkoxide is protonated
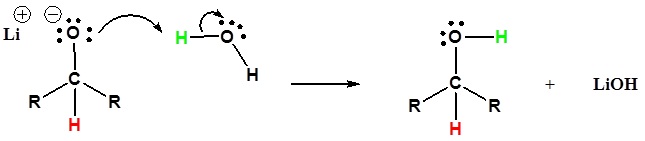
Biological Reduction
Add-on to a carbonyl past a semi-anionic hydride, such equally NaBH4, results in conversion of the carbonyl chemical compound to an alcohol. The hydride from the BHiv - anion acts every bit a nucleophile, calculation H- to the carbonyl carbon. A proton source can then protonate the oxygen of the resulting alkoxide ion, forming an alcohol.

Formally, that procedure is referred to as a reduction. Reduction generally ways a reaction in which electrons are added to a compound; the chemical compound that gains electrons is said to be reduced. Considering hydride can exist thought of as a proton plus two electrons, nosotros can think of conversion of a ketone or an aldehyde to an alcohol equally a two-electron reduction. An aldehyde plus two electrons and 2 protons becomes an alcohol.
Aldehydes, ketones and alcohols are very common features in biological molecules. Converting betwixt these compounds is a frequent event in many biological pathways. However, semi-anionic compounds like sodium borohydride don't exist in the jail cell. Instead, a number of biological hydride donors play a similar role.
NADH is a common biological reducing agent. NADH is an acronym for nicotinamide adenine dinucleotide hydride. Insetad of an anionic donor that provides a hydride to a carbonyl, NADH is actually a neutral donor. Information technology supplies a hydride to the carbonyl under very specific circumstances. In doing then, information technology forms a cation, NAD+. However, NAD+ is stabilized by the fact that its nicotinamide ring is aromatic; it was non aromatic in NADH.
Reduction of Carboxylic Acids and Esters
Carboxylic acids tin be converted to oneo alcohols using Lithium aluminum hydride (LiAlH4). Note that NaBH4 is not strong enough to convert carboxylic acids or esters to alcohols. An aldehyde is produced as an intermediate during this reaction, only information technology cannot exist isolated considering information technology is more reactive than the original carboxylic acid.
![]()
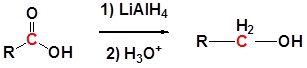
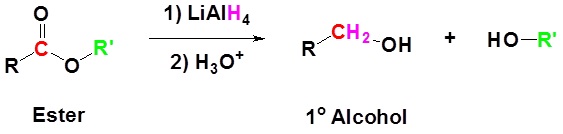
Esters can be converted to 1o alcohols using LiAlH4, while sodium borohydride (\(NaBH_4\)) is not a strong enough reducing agent to perform this reaction.
Exercises
Questions
Q17.4.i
Give the aldehyde, ketone, or carboxyllic acid (there can be multiple answers) that could exist reduced to form the following alcohols.
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
Q17.4.two
Given the post-obit alcohol, draw the construction from which information technology could be derived using just NaBH4
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
Solutions
S17.four.1
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
S17.4.two
Note, NaBH4 is only a strong enough reducing agent to reduce ketones and aldehydes.
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
Source: https://chem.libretexts.org/Courses/Athabasca_University/Chemistry_360:_Organic_Chemistry_II/Chapter_17:_Alcohols_and_Phenols/17.04_Alcohols_from_Carbonyl_Compounds:_Reduction
0 Response to "What Carbonyl Compound Would You Reduce to Produce the Following Alcohol?"
Post a Comment